In the aftermath of a national health emergency declaration for Monkeypox, the United States has now decided to split the approved MVA-BN vaccine into five doses in an effort to stretch supply.
Some experts, however, have warned that the plan may backfire if health workers are not sufficiently trained in the intradermal skin-based jab technique – that is supposed to provoke a more powerful immune reaction with a much smaller vaccine dose.
On Tuesday, US Health and Human Services Secretary Xavier Becerra issued a 564 determination, granting the US Food and Drug Administration the power to issue an emergency use authorization for vaccines. This gives the FDA permission to change the way the MVA-BN vaccine, made by Danish company Bavarian Nordic, is administered.
‘A game-changer’
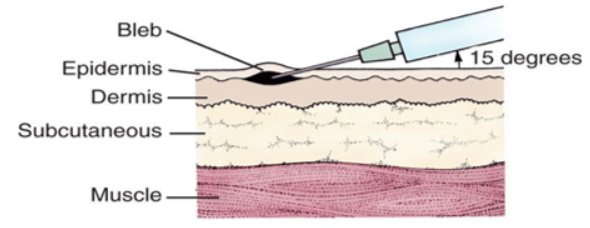
The fractional dose strategy, also known as dose sparing, means that the vaccine will be delivered just under the outermost layer of skin, the dermis, as compared to the typical subcutaneous injection, which penetrates more deeply.
This more shallow approach can potentially generate a stronger immune response with smaller amounts of vaccine than under the skin or into a muscle, allowing a one dose vial to be stretched to 5 separate doses.
Robert Fenton, the newly appointed White House Monkeypox response coordinator, called the move “a game changer.”
“It’s safe, it’s effective, and it will significantly scale the volume of vaccine doses available for use across the country,” Fenton said during a press conference.
The US National Institute of Allergy and Infectious Disease (NIAID) will, however, be designing a clinical trial to compare the fractional dose regimen and a single, full-dose regimen to the standard two-dose approach.
Critics question decision saying knowledge of efficacy limited
Some experts, however, have raised concerns about the decision to adopt fractional dosing before further study is done, citing both limited research findings on the efficacy for monkeypox vaccine as well as the additional training that health workers need to deliver the vaccine.
Health professionals in the US do not have a lot of experience giving vaccines via the intradermal route, and would need to be trained, noted Philip Krause, former deputy director of the US Food and Drug Administration in an opinion piece published Tuesday in STAT.
“Vaccines are not typically given intradermally in the US, and there is little margin for error,” said Krause and co-author Luciana Borio of the Council on Foreign Relations.
“Mistakes could cause a lower dose of the vaccine to be delivered deeper than intended, with likely lower effectiveness. A hasty decision to try an unproven and risky strategy to stretch the existing vaccine supply may interfere with developing a national plan to quell this outbreak,” they concluded.
National Coalition of STD Directors also questions decision
The National Coalition of STD Directors, which represents sexual health clinics that have been at the forefront of the monkeypox response, also questioned the decision.
“To implement a change of course like the administration is proposing requires additional staff, training, supplies like new syringes, and ultimately trust — which has been slowly chipped away each and every time the vaccine strategy changes,” Executive Director David C. Harvey said in a statement.
“We have grave concerns about the limited amount of research that has been done on this dose and administration method, and we fear it will give people a false sense of confidence that they are protected. This approach raises red flag after red flag, and appears to be rushed ahead without data on efficacy, safety, or alternative dosing strategies.”
In fact the intradermal procedure is used for a few other vaccines – including hepatitis B vaccines in adults as well as the BCG tuberculosis vaccine in infants as well as children. However, the BCG vaccine is not widely administered in the United States, while the hepatitis B vaccine is primarily administered to groups at risk of infection, such as health workers.
Global supply shortage due to Bavarian Nordic production plant closure
The decision to shift to dose sparing was taken amidst a global shortage of monkeypox vaccines, due to planned closure of Bavarian Nordic’s European production plant until late 2022.
Notably, Bavarian Nordic is the manufacturer of the world’s only approved vaccine for monkeypox. With only 16.4 million doses of its MVA-BN vaccine available worldwide, it remains unclear how the company plans to meet rising demand, following a declaration from the World Health Organization that monkeypox is a global health emergency of international concern.
As of 10 August, there were now 29,833 confirmed cases reported globally, with 4764 cases newly reported in the last week. Over 17,500 cases – almost two- thirds – have been reported in the WHO European Region, which is considered at high risk for monkeypox, by the WHO.
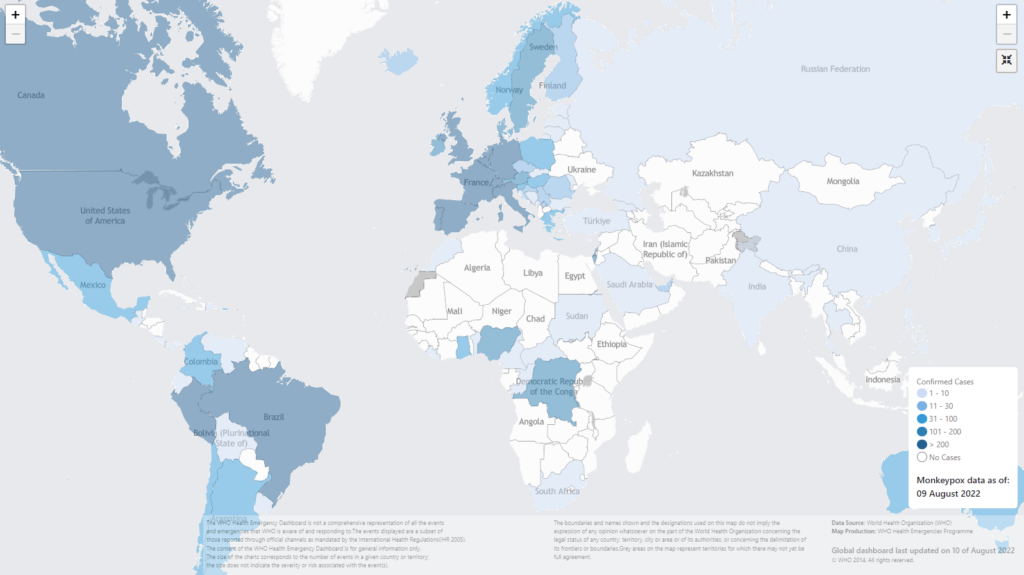
The US has by far the largest case load, reporting 7491 cases so far. Over 600,000 doses of the vaccine have been distributed to state and local jurisdictions, according to federal health officials. However, they have estimated that 1.8 million Americans could be directly at risk from the virus and would thus be candidates for immunization with the two-dose vaccine.
Image Credits: Star919News/Twitter , https://mvec.mcri.edu.au/references/intradermal-vaccination/, WHO .
