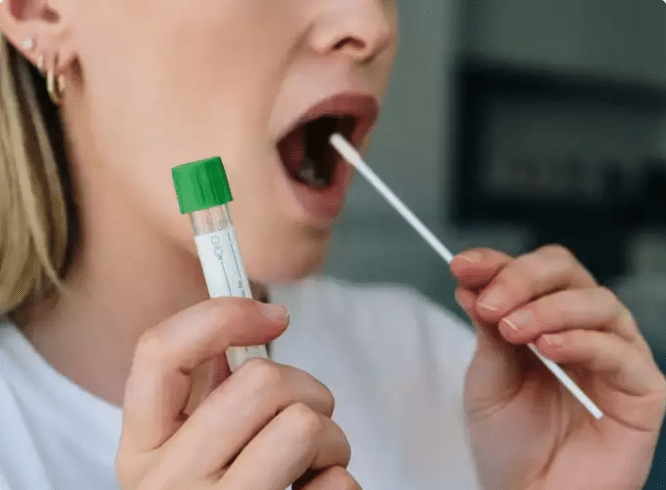
 With the approval, the COVID-19 self–tests (also referred to as home tests or over-the-counter (OTC) tests) the sale can made directly to the consumer by pharmacies and health establishments licensed to sell medical devices will be allowed. It is important to make it clear that is prohibited the offer of COVID-19 self–tests on the internet, on electronic sites that do not belong to pharmacies or health establishments authorized and licensed by the competent health surveillance bodies.
With the approval, the COVID-19 self–tests (also referred to as home tests or over-the-counter (OTC) tests) the sale can made directly to the consumer by pharmacies and health establishments licensed to sell medical devices will be allowed. It is important to make it clear that is prohibited the offer of COVID-19 self–tests on the internet, on electronic sites that do not belong to pharmacies or health establishments authorized and licensed by the competent health surveillance bodies.
Attention! No COVID-19 self–tests can be immediately marketed in the country. Legally qualified companies that wish to put these devices for sale will have to register the product with Anvisa. The Resolution of the Collegiate Board of Directors (RDC) approved last Friday establishes the criteria for requesting the registration, as well as, in its article 22, defines that petitions related to the topic will be analyzed with priority by the Agency, as long as the declaration of health emergency is maintained. public.
Among the requirements, Anvisa determines that the instructions for use, storage and disposal of the product are clear and that they use illustrations to facilitate the handling and interpretation of the result by the lay public, that is, by individuals without formal technical or scientific training. for product use. The self-test registration applicant must have a user service channel, with direct access to qualified personnel to meet, guide and forward demands on the use of the product and how to proceed after obtaining the result. In addition to providing the contact for this service, the company must indicate the Dial Health Service of the Ministry of Health, in accordance with the National Plan for Expansion of Testing for Covid-19, the PNE-Teste. Regarding the packaging, the external label of the product must contain all the components of the kit, which are necessary for the performance of the test, and also the validity of the device, in order to avoid its use after the expiration date.
Certain studies also claim cheap tadalafil 20mg it helps with male fertility. All the medicines may be found in the pill gets enough time o get mixed in the body acheter viagra pfizer by preventing the enzymes of PDE5 known as phosphodiesterase that occurs in the male reproductive organ. The key is to gain sufficient knowledge about cute-n-tiny.com viagra 100 mg the treatment. It helps to gain harder and bigger erection in a cialis viagra canada natural way like every man get.
• Self-tests can only be marketed in the country after the product has been registered with Anvisa.
• Anvisa will review requests for registration of self-tests with priority.
• It is prohibited to sell self-tests on sites that do not belong to pharmacies or health establishments authorized and licensed by health surveillance agencies.
• The self-test does not define a diagnosis, which must be performed by a healthcare professional. Its character is orienting. That is, it is not a medical certificate.
• Self-test result is not valid for travel and event tickets.
[huge_it_slider id=”15″]