This first release of myMedidata includes a research-based COVID-19 symptom tracker, that is designed to support research studies and advance scientific understanding of the virus. It will be made available to Medidata customers free of charge. This app will provide sponsors the ability to collect symptoms directly from research participants who may not otherwise be able to continue with traditional site visits. It also allows researchers to recruit large registries of individuals to monitor their health status with respect to COVID.
“It is critical for the life sciences industry to understand the effects of SARS-CoV-2 on patients, and to find ways to forge ahead with research across all therapeutic areas, from COVID-19 to cancer,” said Glen de Vries, co-founder and co-CEO, Medidata. “The early launch of myMedidata allows sponsors to gather patient-reported COVID-19 symptoms in ongoing and new research programs. We are providing this capability on the Medidata platform free of charge, in response to the pandemic crisis.”
myMedidata provides the industry’s single most comprehensive, integrated tool set for all aspects of patient-centered research. These include eConsent (an electronic patient consent system for clinical trial participation), eCOA (electronic clinical outcomes assessment) / ePRO (electronic patient-reported outcomes), Wearable Sensors (collecting data from biosensors and wearable technology) and Virtual Trials.
[huge_it_slider id=”15″]This virtualization of clinical trials – using patient-facing technologies to allow clinical research to be conducted remotely – is essential in the current healthcare environment. A recent White Paper, COVID-19 and Clinical Trials: The Medidata Perspective, demonstrates the effect of lock-downs and quarantines on new patients entering clinical trials for actively recruiting studies, and how digital solutions can mitigate those issues to support the continuation of those studies.
“We need to do everything we can to help patients continue their care in existing clinical trials. We can’t allow the public health threat caused by COVID-19 to create another set of threats for these patients,” added de Vries. “myMedidata is a crucial step in keeping patients involved and supported, regardless of their location.”
myMedidata was built using insights generated by the company’s Patient Centricity by Design framework, where patient advocates regularly engage as a part of the Medidata software design and development life cycle. The new platform provides patients the opportunity to view their own clinical data (current and historical) and increases their engagement with the study team.
“Therapeutics and vaccines cannot be tested without the most critical components in clinical research: The patients, and their ability to participate,” said Anthony Costello, senior vice president, mHealth, Medidata. “Making research more patient-centric and giving patients the ability to virtually access and actively engage in their own health care needs to be the new normal.”
Medidata is a wholly-owned subsidiary of Dassault Systèmes, which with its 3DEXPERIENCE platform is positioned to lead the digital transformation of life sciences in the age of personalized medicine with the first end-to-end scientific and business platform, from research to commercialization.
Key Facts
| News Category |
Other Product News |
| Company |
Dassault Systemes SE
Medidata Solutions Inc |
| Country |
Global
Europe > France
North America > United States of America |
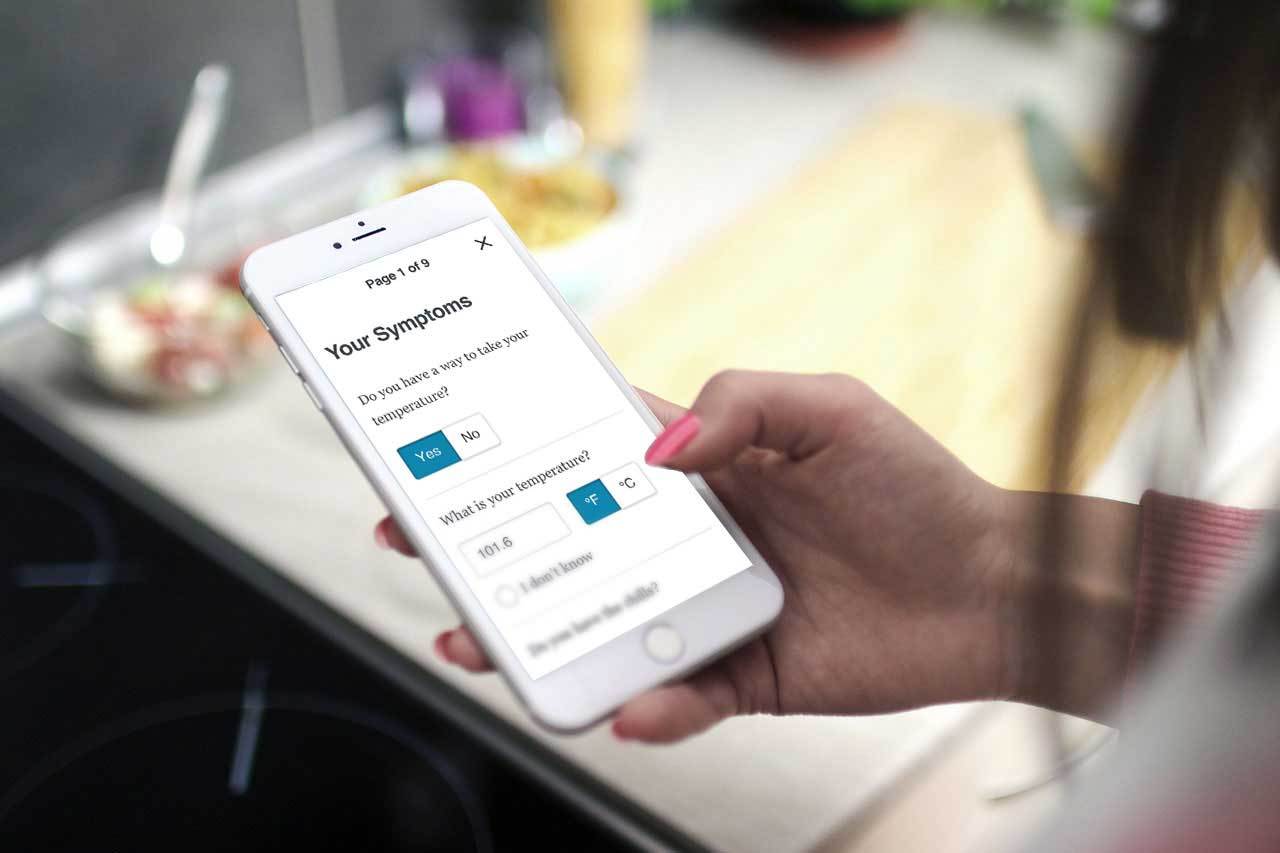 Medidata, a Dassault Systèmes company, announced the launch of myMedidata, an advanced, intuitive platform for patients to enable flexible participation in clinical trials for new medicines and vaccines. myMedidata provides a unified experience for patients, encompassing all of the capabilities of Medidata’s industry-leading, regulatory-compliant Rave platform and Patient Cloud tools.
Medidata, a Dassault Systèmes company, announced the launch of myMedidata, an advanced, intuitive platform for patients to enable flexible participation in clinical trials for new medicines and vaccines. myMedidata provides a unified experience for patients, encompassing all of the capabilities of Medidata’s industry-leading, regulatory-compliant Rave platform and Patient Cloud tools.