
Top executives at Bavarian Nordic, which makes the world’s only monkeypox vaccine, avoided news media in the face of a worldwide shortage of their product. But SIGA, the company behind the antiviral treatment Tecovirimat (TPOXX™), has not been so shy. In an exclusive interview, SIGA’s CEO, Phillip Gomez, says the small, speciality firm is prepared to rapidly scale up its production and support clinical trials testing its efficacy in Africa, Europe and North America, and that it also is ready to strike procurement deals with low- and middle-income countries through global health channels. The catch? The drug, while effective in animals, is only now undergoing its first human efficacy trials.
SIGA is not the kind of pharmaceutical firm that typically finds itself in the global spotlight. Employing 39 full-time employees, the company still functions a bit like the start-up that it was in 1995, with all of its research, development and manufacturing done by external collaborators and contractors.
As holders of the patent for Tecovirimat (TPOXX™) – the only monkeypox treatment approved by European authorities, and on track for authorization in the US – the firm is now poised to become a key player in the global response to the monkeypox epidemic.
SIGA’s CEO Dr. Phillip Gomez seems to be riding the global wave of interest in the drug with apparent ease.
And while the closure of Bavarian Nordic’s manufacturing facility in Denmark led to a worldwide shortage of monkeypox vaccines, SIGA is ready to assume a critical role in fighting the global outbreak, Gomez told Health Policy Watch in a recent interview.
Armed with a secure domestic supply chain, on-hand inventory, and contracts with four US manufacturers equipped to accommodate growing demand, the New York-based company says it is set to quickly scale up production and delivery of its antiviral treatment at a time when patients around the world could greatly benefit from its extended rollout.
But there’s a catch. No published data yet exists on its efficacy in humans.
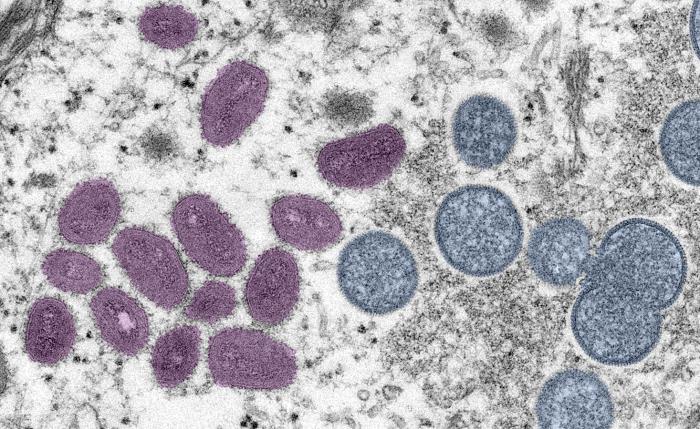
Although the drug’s safety has been established in trials with healthy volunteers, efficacy against monkeypox has only been demonstrated in animal models.
In the United States, tecovirimat first was authorized for use as an oral treatment against smallpox in 2018 under the US Food and Drug Administration’s Animal Efficacy rule, a pathway to approval for treatments of diseases that are so rare, or deadly, that human efficacy studies would be impossible or unethical.
But TPOXX is still not approved by FDA for use against monkeypox. It is thus being made available only for immunocompromised people and other vulnerable groups under the FDA’s expanded use protocol. Activist groups complain of bureaucracy to get access to the drug despite a recent FDA easing of criteria. In contrast, the European Medicines Agency (EMA) approved the drug in 2021 for broad use against the three key orthopox family viruses: monkeypox, cowpox, and smallpox.
Meanwhile, the first real human trial of the drug, which began last year with 14 volunteers in the Central African Republic is due to publish initial results soon. In response to the current crisis, 10 much larger clinical trials are in the works across Europe, North America and Africa in collaboration with the drug manufacturer.
But the results of those trials, even if expedited, remain weeks if not months away.
“Our model for efficacy was monkeypox in monkeys, where we saw greater than 95% protection in a lethal challenge model,” Gomez said of the critical animal study, published in 2018 in the New England Journal of Medicine. “With the monkeys days away from death, our drug was able to treat them, and they had rapid resolution of symptoms.”
Altogether, four studies on TPOXX’s efficacy in infected monkeys consistently showed that the drug reduced viral load, lowered the period of viral shedding, accelerated resolution of the infection, and, in the cases of critically ill animals, averted their death.
The hope, both at SIGA and in affected communities around the world, is that these results will translate into similar impacts for people.
If the antiviral agent can reduce the lengthy period of infection, which can extend from two to four weeks, it also could have the knock-on effects of curtailing transmission of the infection to other people and reducing hospitalizations for often-painful lesions.
“Our belief, or at least our hypothesis, is that treating anyone who’s been infected would shorten their duration of symptoms and reduce viral shedding,” Gomez said. “We think this could be an important contribution to controlling the current outbreak.”
Ramping Up to 600,000 Courses Annually Is Possible

As communities with monkeypox hotspots clamour for solutions, Gomez said SIGA anticipates nearly doubling its production this year.
“In 2020 and 2021, we delivered about 363,000 courses each year to the [US] Strategic National Stockpile, and we do believe we can ramp up to about 600,000 courses,” he said. “There will be a lead time depending on the volume of ramp-up. We can’t do it overnight, but we are in a good position.”
In contrast to Bavarian Nordic, which was caught at the outset of the global health crisis with their manufacturing plant closed, SIGA seems to be poised to ride the crest of the crisis thanks to a combination of luck and good planning design.
“We had anticipated deliveries coming up in the next couple of years given the European Medicines Agency (EMA) approval,” Gomez explained, referring to EMA’s 2021 approval of the drug for smallpox, monkeypox and cowpox.
The company also learned valuable lessons from dealing with the COVID-19 pandemic’s disruptions to global supply chains.
“Supply chain challenges led us to doing a lot of advance production,” he said. “The good news is that it is a small molecule drug; the supply chain is in the US; we have ongoing production, and we have inventory”.
Gomez said the company is responding to many orders and trying to catch up, especially in Europe, the Middle East, and Asia, so that it can both provide the drug and be able to distribute it to patients.
“It all depends on where the outbreak goes, but certainly in the short term we think we’ll be able to meet demand,” he said, adding that the public sector needs to do its part too.
“It is important for governments to plan for the worst case scenario, and hopefully work with us to plan for that as well,” said Gomez. “The better the global health community can plan ahead and think about where this is going, rather than what cases they have right now, the better.”
‘We certainly are’
Prior to the monkeypox outbreak, the US government stockpiled 1.7 million courses of the antiviral agent as preparation for a smallpox outbreak or attack, which was the original reason for developing TPOXX as part of Project Bioshield.
Canada, and one unnamed country in the Asian-Pacific were the only other nations to order and stockpile the drug while others failed to think ahead, according to SIGA’s published records.
“We have been talking to countries in Europe for years,” Gomez said, “but nobody thought it was of significant enough concern that they actually stockpiled the drug in advance.”
European authorities, facing criticism for their failure to stockpile smallpox and monkeypox vaccines, are holding discussions with SIGA for a bulk procurement order for the bloc, Gomez said. This was independently confirmed by the EU’s Health Emergency Preparedness and Response Authority (HERA).

“For us, the greater question is: How large does this get, and are governments willing to make investments to make sure that we are able to continue to ramp up?” he asked.
Gomez indicated SIGA also is open to working on a procurement plan for countries unable to outbid high-income economies through traditional market channels. Low- and middle-income countries often get pushed to the back of the line with new drug innovations, even when it’s for a disease like monkeypox that is endemic to a developing region.
“We certainly are,” Gomez said, noting that SIGA has already held discussions with WHO, the Gates Foundation, and CEPI — all agencies headquartered in Geneva. “We’ve predominantly been working with the WHO on thinking about how this could happen, and are very open to the idea.”
Gomez’s experience in public health issues seems to have influenced the company’s DNA. He has sat on Gavi’s board and worked with the Bill and Melinda Gates Foundation to get vaccines and pharmaceuticals into high-risk developing world communities during a stint in the global health division of PriceWaterhouseCoopers. During his years at the US National Institutes of Health, where he worked on HIV, SARS, Ebola and West Nile virus, Gomez was involved in the interface of emerging diseases and R&D into new vaccines and treatments.
“After 9/11 and the [2001] anthrax attacks, we did the first SARS vaccine, which at the time was the world record for vaccine development,” Gomez recalled. “As our colleague, Tony Fauci likes to say, it was the discovery of the [SARS] virus to Phase I [trials] in two years. In retrospect, we should have been worried about coronaviruses more broadly in 2003, but obviously hindsight is 20/20.”
First human trial launched serendipitously before crisis began
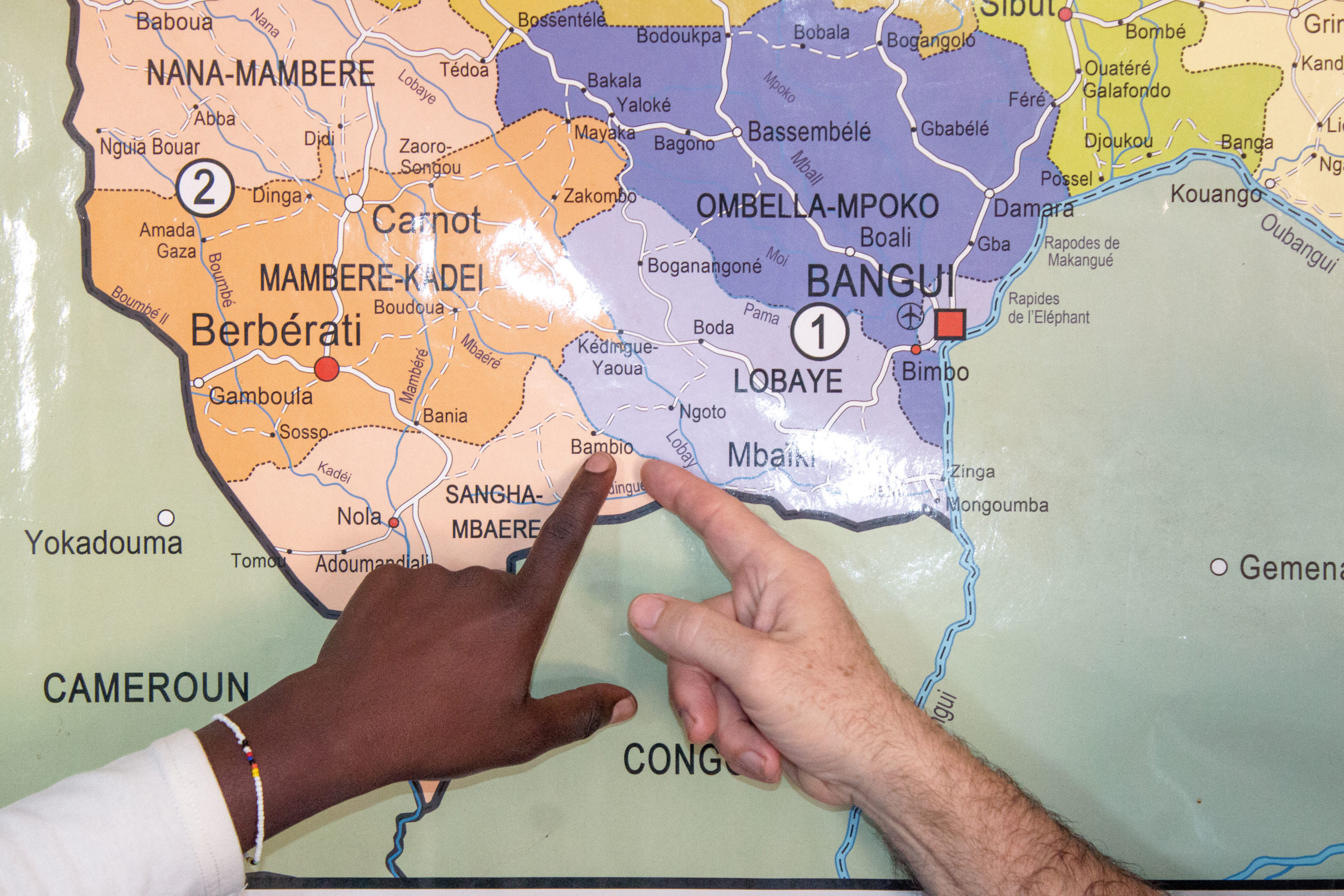
Serendipitously, the first human trial of the drug launched in July 2021 in the Central African Republic. An expanded access study is occurring in remote communities where the most dangerous virus variant, Clade 1, circulates seasonally, often as spillover events passed by infected animals to humans.
Although the trial is small, involving only 14 volunteers who became infected and agreed to take part, initial findings will hopefully be published soon as a “short communication” about cases treated, says Piero Olliaro, an Oxford University professor of poverty-related infectious diseases who is co-leading the trial with Emmanuel Nakoune, scientific director of Institut Pasteur Bangui.
SIGA agreed to provide up to 500 courses of TPOXX for the study, which is being conducted as part of a partnership between SIGA, Oxford and CAR’s Ministry of Health.
In an interview with Health Policy Watch, Olliaro wouldn’t say what the team’s findings are until they are published. Reading between the lines, however, the overall impression remains positive.
“It is very rare that in a crisis, we find ourselves with both a drug and a vaccine,” said Olliaro. “We must use the tools we have at our disposal, but it is just as critical that we study what they do in their deployment in order to understand how they work, and what we can expect from them.”
Until randomized-control trials (RCTs) are conducted, seasoned experts like him remain cautious.
“There has only been data on three patients treated for monkeypox in the world, plus the 14 patients we have treated in CAR,” he said. “There is very little information – actually close to no information – about the period of infection of a person on tecovirimat versus not on tecovirimat.”
Olliaro says this is one of the questions he hopes the trial will address. “Hopefully,” he said,”more trials are set up around the world to answer this key question: Can we reduce the period of infection through treatment?”
At the same time, he stresses that trials in Central Africa, where most people are infected with the more deadly Clade 1 of the virus, can have a very different set of implications than those undertaken elsewhere, where Clade 2 is predominant.
“One out of 10 people infected with Clade 1 are expected to die,” said Olliaro. “It is a fundamentally different outbreak to what we are seeing in Western countries.”
New trial planned in Democratic Republic of Congo (DRC)

SIGA is collaborating in the first randomized-control clinical trial (RCT) of the drug due to launch soon in the DRC. The trial, in collaboration with the DRC’s Institut Nationale de Recherche Biomedicale (INRB) and the US National Institute of Allergy and Infectious diseases (NIAID), will take place in various regions across the country where the disease has been endemic for more than 50 years.
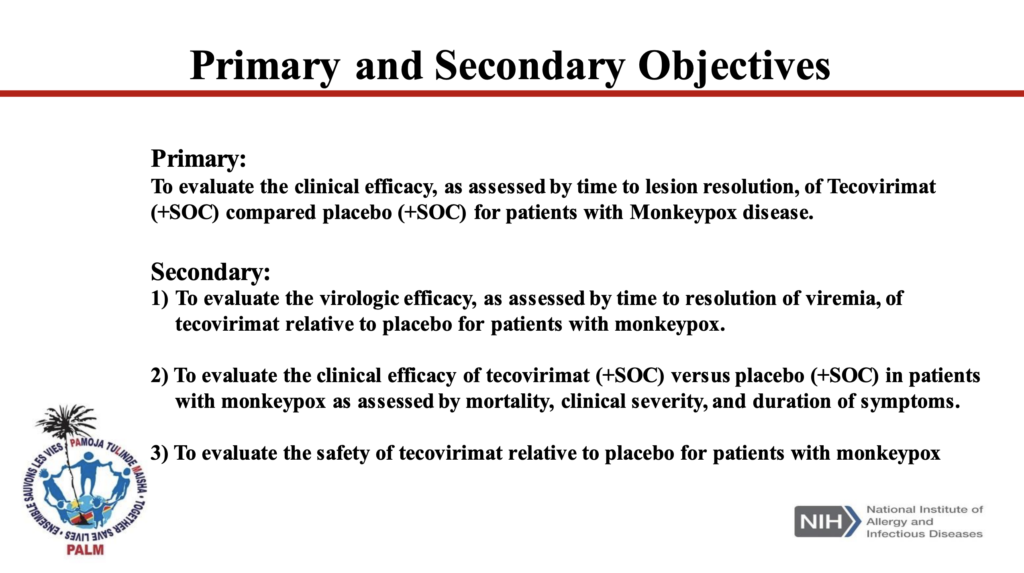
Set to begin in 2022, the trial is significant because it will mark the first RCT of TPOXX, considered the gold standard in clinical research. And here, as in CAR, the dominant monkeypox variant has a mortality rate of 10%.
The trial in three central DRC provinces will follow infected patients for 58 days, measuring the efficacy of a 28-day oral treatment with TPOXX in comparison to a placebo. Its primary indicator of efficacy is time required for lesions to heal. Patients at increased risk from their participation in the study would be excluded.
The ethics of undertaking such a trial when the disease can also turn deadly are complex, but unequivocal findings in one of the world’s most affected regions would provide strong proof of the drug’s efficacy, paving the way to saving lives down the line.
“I believe a placebo-controlled trial is acceptable because we need definitive evidence that tecovirimat works or doesn’t,” Olliaro said.
Tackling monkeypox as a neglected disease of poverty
For SIGA, the trials in CAR and the DRC fulfill a dual purpose, Gomez says.
On the clinical side, they will provide the first systematic evidence about efficacy in humans. That includes whether the antiviral speeds the resolution of infectious lesions, reduces mortality or has any unsafe or adverse effects in the presence of other co-infections such as HIV.
But taking part in studies in Africa also reflects the company’s desire to wield the drug against a neglected disease of poverty, stresses Gomez.
“Part of the reason we did the Oxford study was we wanted this drug to get to the populations that are impacted,” he said. “We knew it’d be important to get data in Central Africa. … We are doing this work in parallel because it is critical for everyone.”
Up to 10 clinical trials in Europe, the US and Africa are in the works
As monkeypox cases swell worldwide, so have the plans for studies of the drug in higher-income countries that are mostly seeing cases of Clade 2 of the virus.
Clade 2, which circulated endemically in Nigeria and other West African countries before breaching international borders this year, is considered far less deadly but is claiming victims, too.
The first five fatal cases outside of endemic African regions were recorded this month in Spain, Brazil, India, and Peru.
About 10% of these cases lead to such painful lesions that hospitalization is required, showing the urgency for rapid clinical evaluations and wider rollouts of the antiviral.
Altogether, SIGA is supporting the launch of up to 10 clinical trials in Africa, Europe and North America “to formally assess the effectiveness of TPOXX to treat and/or prevent monkeypox in human patients,” its chief scientific officer, Denis Hruby, told investors on a 7 August call. “These include both multinational observational studies as well as placebo-controlled research clinical trials.”
He says the company supports plans for more clinical trials in Europe and North America.
In the US, one large-scale RCT is scheduled to begin this fall to study the efficacy of TPOXX in adults infected with monkeypox, including people living with HIV. It will be managed through a partnership between the NIAID (part of NIH), and the AIDS Clinical Trials Group, a research network founded in the 1980s to assess the safety and efficacy of HIV antiviral treatments.
This is important since leading US experts have signaled that full approval for TPOXX’s use against monkeypox should not be granted until RCT data from the US is collected.
“Without data from RCTs, we will not know whether tecovirimat would benefit, harm, or have no effect on people with monkeypox disease,” according to four senior officials from the US Centers for Disease Control and Prevention (CDC), NIH and FDA in a 3 August perspective published in the New England Journal of Medicine.
Researchers in Canada, Europe, and the United Kingdom are preparing protocols for studies of infected people who are already receiving the drug. Many of the trials, however, are observational.
At a recent WHO research symposium on monkeypox, researchers pointed to the practical and ethical issues associated with administering placebos to people already ill, often painfully so, and thus clamouring for real treatment – not a placebo.
“I don’t think that the randomization of a list approach would really be feasible,” Canadian Public Health Agency researcher Matthew Tunis said of his nation’s vaccination drive, which was a collaboration with LGBTQI+ activists groups. “It’s been much more driven at the community level.”
SIGA also is working with the US Department of Defense to conduct two post-exposure prophylaxis clinical trials.
The company hopes to complete the active [recruitment] phase of these trials by as early as September.
“There’s a pan-European protocol being put in place. Canada is working on one, and the US is working on one,” Gomez said. Unlike observational studies in hospital settings where only extremely ill patients receive the drug, he said, the “human data will really come with the outpatient trials” which can safely include placebo-controlled arms.
Anecdotal evidence

Until these studies are completed, however, the only published evidence about efficacy in people involves anecdotal reports of people able to obtain treatment in monkeypox hotspots like New York City.
Nephi Niven Stogner, who is 39, struggled to secure access to the antiviral for two weeks before clearing the high bureaucratic hurdles in the US. He told the New York Times that within 24 hours of receiving the drug, his “lesions went from swollen and red, to flat dark spots.”
As a result of activist pressure, the CDC and FDA recently removed some of the many restrictions on prescribing tecovirimat, although the drug is prescribed mainly on a case-by-case basis to people at high-risk of severe disease.
“There’s quite a lot of anecdotal data out there right now,” Gomez said, “but I’d hate to reference anecdotal data until we really know for sure.”
Strong safety profile derived from human trials
Knowledge about the power of TPOXX against infection is evolving, but one thing is certain. It has a strong, tested safety profile for human use, backed up by multiple trials in healthy adults. The 2018 FDA authorisation of the drug for smallpox cites a safety trial involving 449 healthy people.
Three years later, the EMA review of TPOXX as a treatment for smallpox, monkeypox, and cowpox considered a trial of 788 healthy adults who were administered the antiviral course, with no serious adverse effects.
“The overall risk-benefit balance of Tecovirimat SIGA is positive,” the agency noted in its final approval, dated November 2021.
Right Place, Right Time
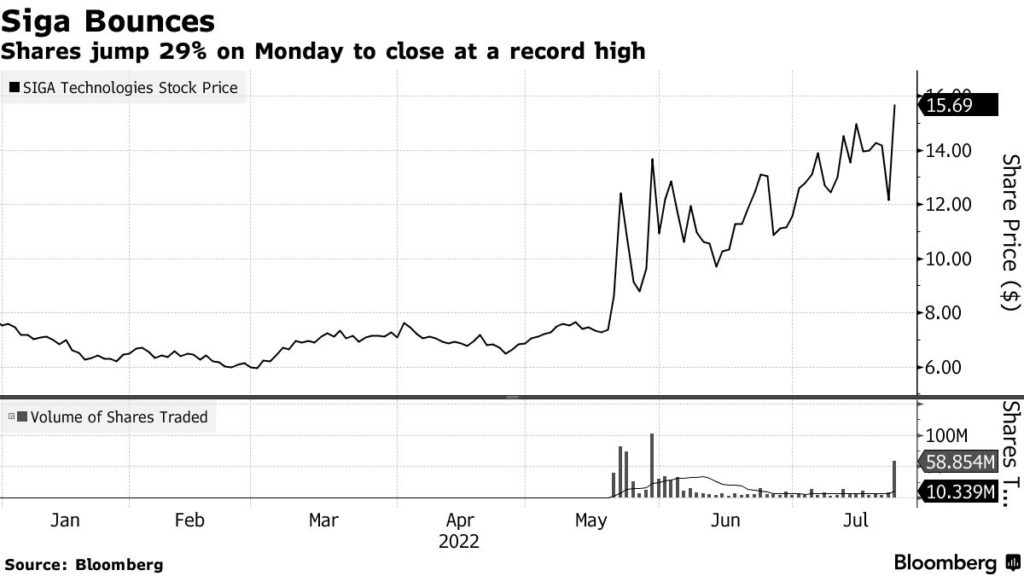
Financially, SIGA has clearly found itself in the right place at the right time. On its August 7th earnings call, Gomez said the company has taken more than US$60 million in new orders since the global monkeypox outbreak began from 10 international jurisdictions. SIGA’s sales for this same period last year were US$13 million.
SIGA disclosed US$41 million of these orders in press releases dated June 23rd and July 12th. Some US$13 million in orders came from two new clients – unnamed countries in Europe and the Asia Pacific – while the remaining US$28 million included Canada (US$26 million), and another unnamed “Asian Pacific” country (US$2 million).
“We have seen an increase in orders [since the WHO’s global emergency declaration]”, Gomez said. “I expect that these increases in demand will continue.”
At the same time, SIGA is not without its critics. Notably, some of the European countries negotiating with the company are not thrilled over the price that has been quoted to them for the drug: reportedly CHF 1,800 per course in Switzerland, £1,500 in the UK, and €2,000 in other parts of the European Union.
That’s in comparison to the United States, where public records show that the government paid around US$300 per course under its BARDA bulk order, and Canada slightly north of US$900 per course.
“Now that many countries want the drug, there is a problem,” observed one European diplomatic source, who asked not to be named.
“Our pricing is volume dependent,” Gomez responded. “It hinges on how many courses are purchased.”
US Invested heavily in drug, SIGA reaps huge benefits
While European countries may criticize the drug’s high cost, it is US government policy to demand best pricing on drugs that it helped develop. In this case, the Biomedical Advanced Research and Development Authority (BARDA) subsidized TPOXX R&D to the tune of US$884 million beginning in 2002 as part of its smallpox defense strategy.
In addition to the subsidies, SIGA reaped significant financial benefits from the partnership.
As a reward for FDA approval of a new drug that treats a neglected “tropical” disease, for which market incentives typically are weak, SIGA received a priority review voucher (PRV). The voucher, which is transferable, allows the holder to cut the line for FDA review of another forthcoming drug, implying a significant monetary value.
SIGA sold the voucher to Gilead Sciences for a lump sum of US$80 million.
The public-private model under which TPOXX was developed has also left SIGA with a de facto monopoly over the monkeypox antiviral market, presenting the company with an immense opportunity to profit from a product developed with public money.
These government-funded subsidies and incentives also give the US the final say in whether SIGA can eventually offer the treatment at a concessionary price to low- and middle-income countries in a bulk procurement deal, Gomez says.
“It’s a little too soon for me to say because I need agreement from the US government, because they essentially demand best pricing [for drugs that they have invested in], but I’m sure they’d be supportive of it,” he said.
Good Intentions Don’t Fix a Broken Model

Despite SIGA’s forward-looking vision, the same push-and-pull mechanisms hampering equality in vaccine access also stand in the way of fair global distribution of tecovirimat.
The largest stockpiles of the antiviral agent are still held by the world’s richest countries, including the US, Canada, and possibly Japan. [SIGA obtained a patent there for the antiviral in 2014, when the drug was perceived primarily as a tool against smallpox]
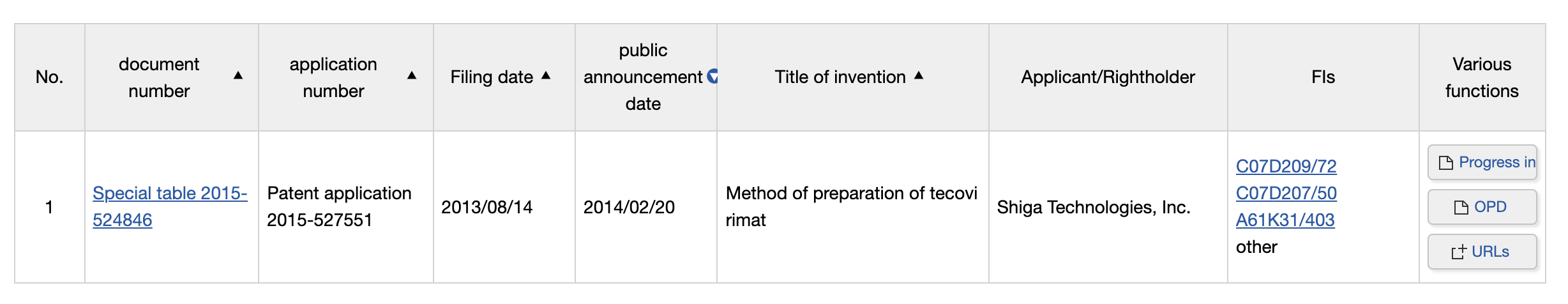
This concentration of treatments in high-income countries, along with chronically weaker health systems lacking the means to rapidly diagnose and treat even serious cases in endemic countries, present a serious challenge to questions of equality of access.
In addition, neither the treatment nor the vaccine alone will work, says Olliaro. “They must be part and parcel of a series of measures, which in this case includes a lot of buy-in from a community of people who have shown in the past that they can do a lot.”
“If you think about it, we are in a situation where there is a single producer for the vaccine, a single producer for the drug, and where the products have been stockpiled by rich countries,” Olliaro told Health Policy Watch.
“There are voices being raised in Africa saying, ‘Wait a second. We’ve been dealing with this for over 50 years, and now all of a sudden there’s all this attention?” he said. “It is a system that solves the problem for wealthy countries, but does not solve the larger one for the rest of the world: treatments are not available for all.”
Fight Against Monkeypox Requires Comprehensive Global Strategy
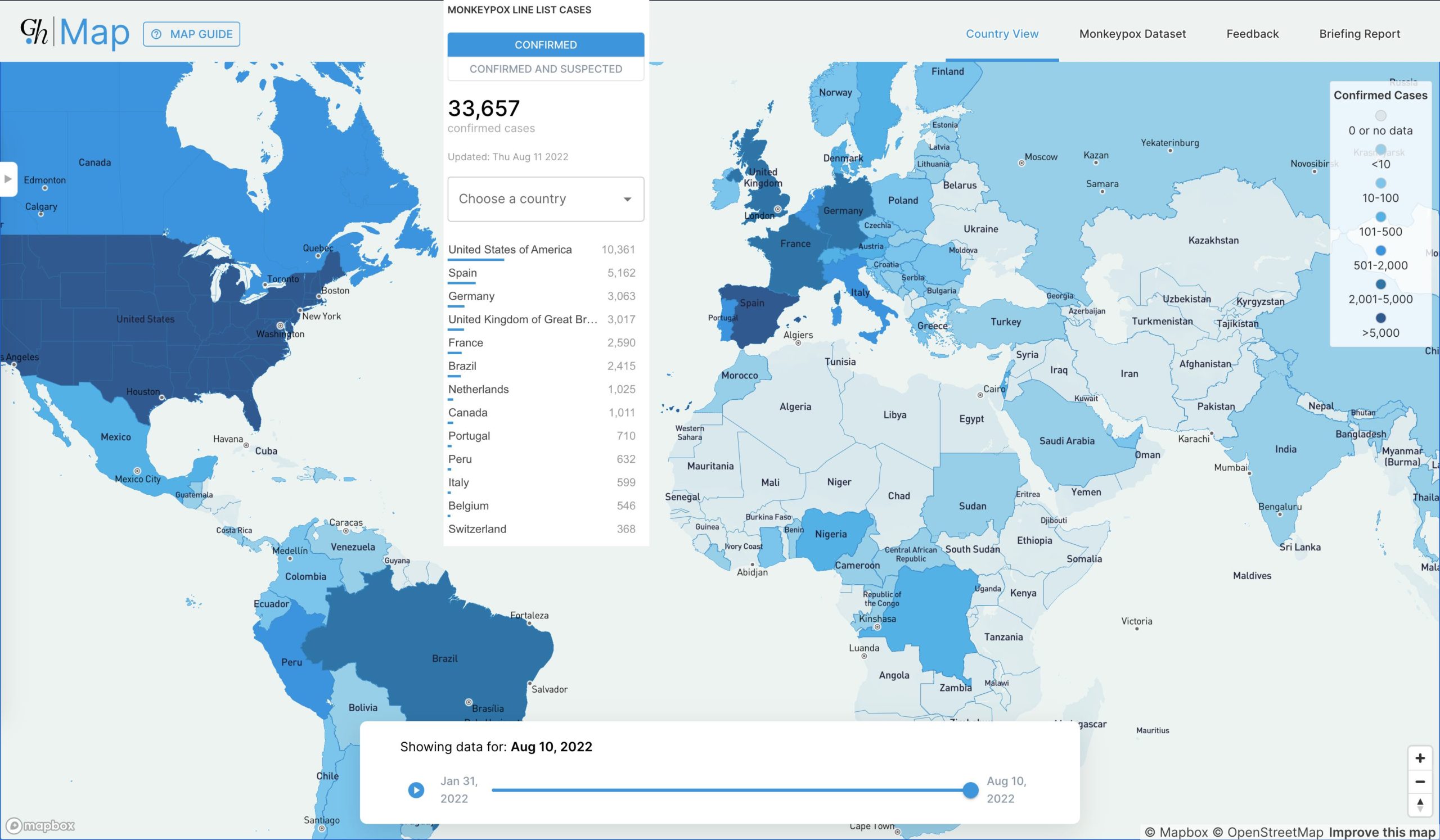
At the time of publication, the confirmed case count was 33,657 infections across 87 countries.
Part 2 of a Health Policy Watch Series on Global Monkeypox Preparedness.
Image Credits: The Hill/Twitter .
